Orthofix Discontinues M6-C™ and M6-L™ Artificial Disc Replacement
Orthofix has officially withdrawn the M6-C™ and M6-L™ artificial disc replacement products from the market.
Why Was the M6 Artificial Disc Discontinued?
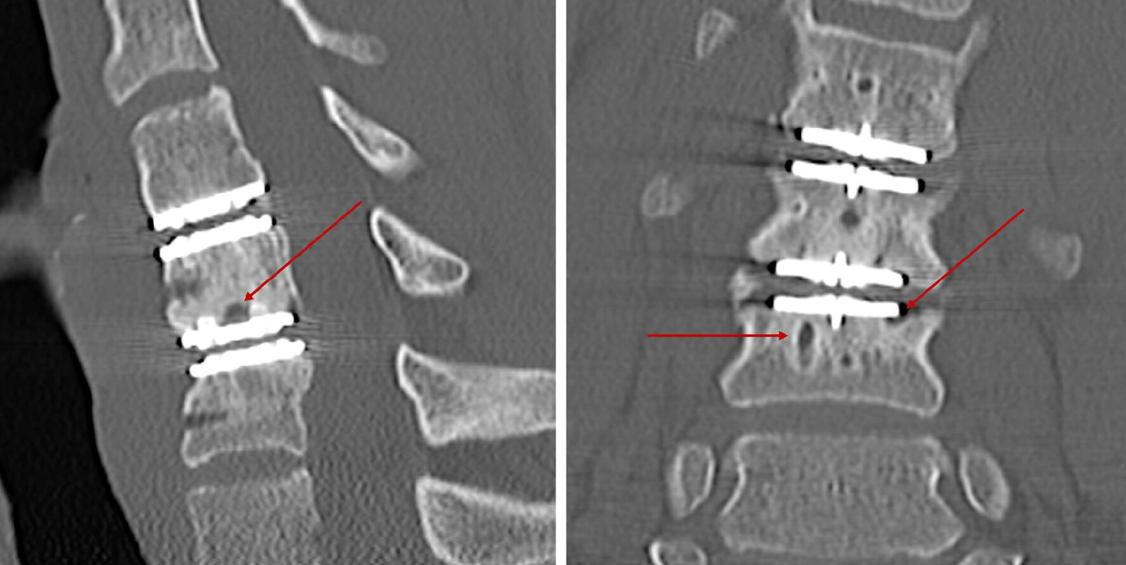
Recent studies have raised serious concerns about the long-term safety of the M6-C cervical artificial disc. According to a study published in the European Spine Journal, up to 34% of patients who underwent M6-C disc replacement developed severe osteolysis (bone loss) requiring revision surgery and spinal fusion.
This alarming failure rate was linked to polyethylene wear debris from the M6-C implant—significantly higher than any other cervical disc replacement on the market.
Key Findings from the European Spine Journal Study:
- 34% of patients experienced severe osteolysis.
- Bone loss led to revision surgery and spinal fusion.
- Polyethylene wear debris was identified as the cause.
What This Means for Patients
If you have received an M6-C or M6-L artificial disc replacement, it’s crucial to stay informed about potential complications. Consult Microspine today to discuss any potential next steps. Fill out the form below or call 602-833-2141.
